Back to "Biological Language Modeling Seminar Topics"
Reference: www.mshri.on.ca/pawson/research1.html
Back to "Protein protein interactions"
PDZ Domains
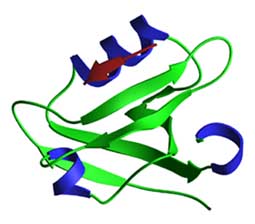
PDZ domains contain ~80-90 residues that fold into a structure with a b-sandwich of 5-6 b-strands and two a-helices. The peptide ligand binds in a hydrophobic cleft composed of a b-strand (bB), an a-helix and a loop that binds the peptide carboxylate group. The peptide binds in an anti-parallel fashion to the bB strand, with the C-terminal residue occupying a hydrophobic pocket. PDZ heterodimers form a linear head-to-tail arrangement that involves recognition of an internal on one of the partner proteins. The figure shows the 3rd PDZ domain of PSD-95 bound to a TKNYKQTSV peptide.
Domain binding and function
type 1 PDZ consensus binding motif:
x-[ST]-x-F-stop (where F is large and hydrophobic) [Reference: Hung AY, Sheng M (2002) PDZ domains: structural modules for ptoein complex assembly. J. Biol. Chem. 277, 5699-5702]
PDZ domains bind to the C-terminal 4-5 residues of their target proteins, frequently transmembrane receptors or ion channels. Reference: Kornau et al. (1995) Domain interactions between NMDA receptor subunits and the postdynaptic density protien PSD-95. Science 269, 1737-1740.
These interactions can be of high affinity (nM Kd). The consensus binding sequence contains a hydrophobic residue, commonly Val or Ile, at the very C-terminus. Residues at the -2 and -3 positions are important in determining specificity. PDZ domains can also heterodimerize with PDZ domains of different proteins, potentially regulating intracellular signalling.
PDZ domain protein |
Binding partner |
Binding Site |
| Post-synaptic Density protein 95 (PSD-95) | NMDA receptor B via PDZ1 and PDZ2 of PSD-95 | IESDV-COOH |
| Kvl1.4 (Shaker-type K+ channel) via PDZ1 and PDZ2 of PSD-95 | VETDV-COOH | |
| neural Nitric Oxide Synthase (nNOS) via PDZ2 | PDZ/PDZ interaction |