
Back to "Homepage of Judith Klein-Seetharaman"
Back to "G-protein coupled receptors"
G-protein coupled receptor Phosphorylation
References for which site becomes phosphorylated first:
Evidence for 343:
McDowell, Nawrocki, Hargrave (1993) Biochemistry 32, 4968-4974
Papac, Oatis, Crouch, Knapp (1993) Biochemistry 32, 5930-5934
Ohguro, Johnson, Ericsson, Walsh, Palczewski (1994) Biochemistry 33, 1023-1028.
Effect of phosphorylation on structure: Dorey et al. (1999)
Without phosphorylation:
19aa-peptide 330-348 solution structure (similar to 33aa- and 43aa-peptide structures, 315-348 and 305-348)
includes a short antiparallel beta-sheet that includes 336, 338 and 343 (see figure below)

Comparison of C-terminus in the rhodopsin crystal structure (1L9H.pdb) and the "full rhodopsin NMR structure (including C-terminus)" (1JFP.pdb) obtained by assembly of structures of individual fragments. Note that the structure of the C-terminus is entirely different from that reported by the same group earlier (Dorey et al., 1999). Earlier structure may have been more similar to crystal structure (as judged by Figure 5 in Dorey et al., 1999, but no pdb file was submitted).
Effect of phosphorylation:
1. at 343
conformational change (twisting of the beta-sheet)
2. at 343, 338, 334
no further conformational change beyond the one observed after phosphorylation at position 343
3. at 334, 335, 336, 338, 340, 342, 343
additional conformational changes. The interstrand hydrogen bonds in the beta-sheet break, but the turn that leads to the antiparallel strands is still observed
Specific distances of C-terminus with respect to rest of cytoplasmic face:
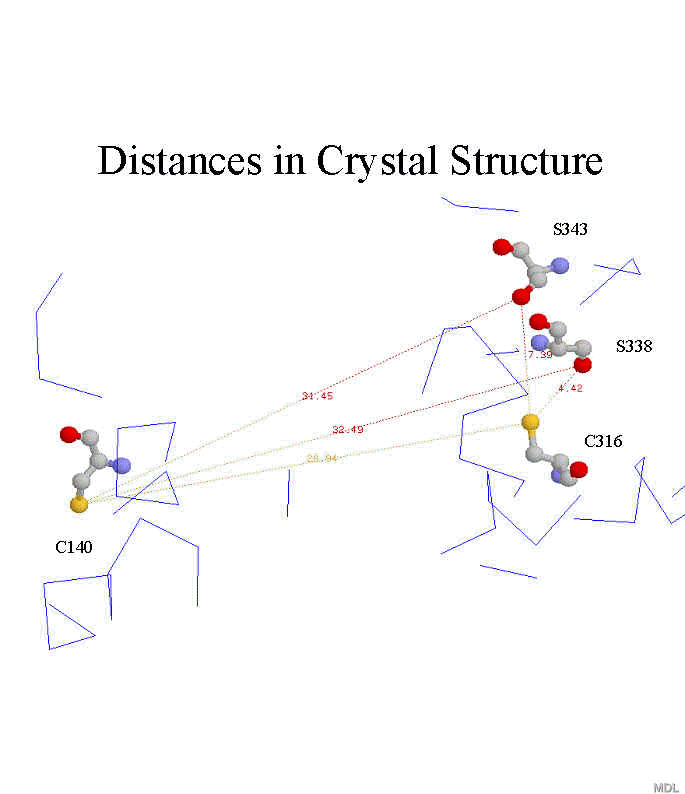
Figure: Distances between cysteine sulfur atoms at 140 and 316 with respect to serine gamma-oxygen of S343 and S338 in crystal structure (1L9H.pdb)
Distance between Cys140 and S338 and/or S343 (Albert et al. 1997):
Method: MAS of phosphorylated rhodopsin in ROS membranes (both S338 and/or S343) with and without EPR spin label at position C140, modeling of the broadening of the 31P resonance by the EPR spin label in terms of distance
Result: MAS spectrum of 31P resonance(s) observable, but no temperature was given in the paper. The distance was determined to be 18+3Å between 140 and 338/343.
Mobility of phosphorylated C-terminus in ROS (Albert et al., 1990):
ROS membranes were phosphorylated to a varying extent of 2-10 P/rhodopsin
MAS of phosphorylated rhodopsin gave rise to new signals in addition to the phospholipid signals, that could be removed by limited proteolysis of the C-terminus
The resonances were very sharp, indicating that at least part of the C-terminus is very mobile (no temperature was given).