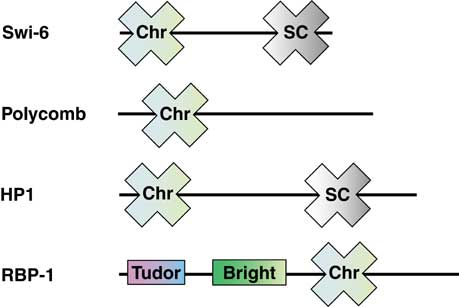
Structure
Structure
The Chromo domain appears as an N-terminal
three-stranded anti-parallel b-sheet which
folds against an N-terminal a-helix. A
conserved series of hydrophobic residues that winds across the face of
the b-sheet is referred to as a ‘sash’. Interactions with partner
proteins are thought to be mediated by the residues within the
hydrophobic sash. The figure shows the structure of the chromo domain
from mouse modifier protein 1. Reference: Ball et al. 1997 EMBO J.
16:2473-2481.